In a significant development, Philips, the healthcare technology giant, has reached a settlement in the United States regarding its recalled DreamStation sleep apnea devices. The company has agreed to pay a staggering $1.1 billion in damages to affected patients, although it maintains that this settlement does not imply any admission of fault.
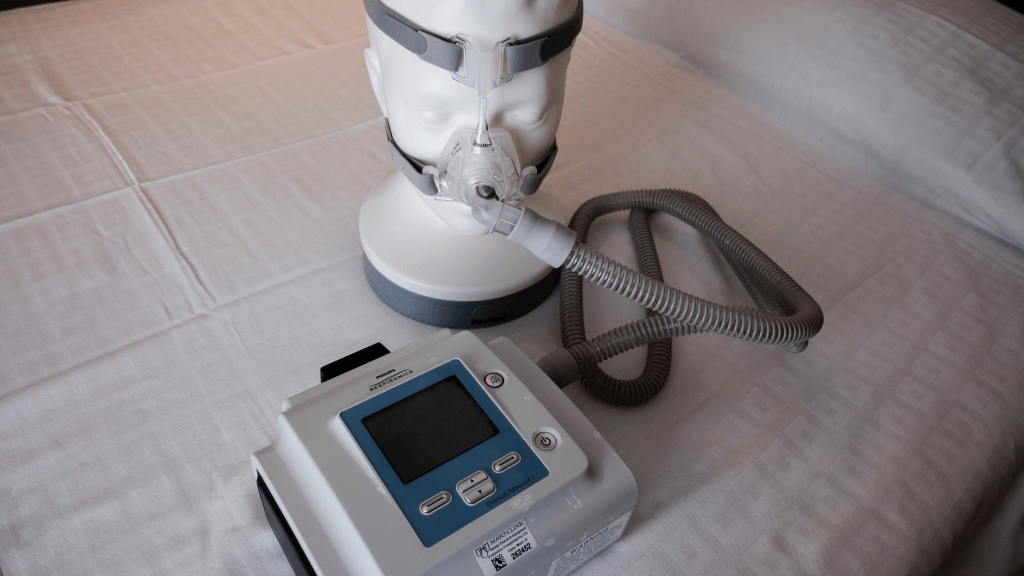
This resolution comes after a tumultuous period following the recall of approximately 5.5 million first-generation DreamStation devices in 2021. The recall was prompted by the discovery that the sound-dampening foam in these machines could disintegrate, potentially posing a risk to users if they used incorrect cleaning agents. Notably, this recall stands as one of the largest in the history of medical devices globally.
The settlement is notably lower than initially anticipated by financial experts. Analysts had projected a figure closer to $3 billion to address the claims of an estimated 60,000 American users affected by the recall. Despite the settlement, Philips continues to face scrutiny, including an ongoing investigation by the U.S. Department of Justice, which could result in further financial penalties.
Under the terms of the settlement, Philips does not accept liability for any injuries caused by the devices. Furthermore, the DreamStation devices are currently not available for sale in the United States, following an agreement with the Food and Drug Administration (FDA).
This development adds to a series of challenges for Philips in the realm of medical devices. In addition to the DreamStation recall, the company faced issues with thousands of ventilators in 2021. More recently, concerns have arisen regarding the Trilogy ventilators, with reports of an error that triggers the empty battery/power failure alarm unnecessarily, potentially halting ventilation in critical care settings.
As Philips navigates the aftermath of these challenges, the settlement serves as a significant step forward in addressing the fallout from the DreamStation recall. However, the company’s ongoing legal and regulatory issues underscore the complexities involved in ensuring the safety and reliability of medical devices in the healthcare industry.












0 Comments